The following video is intended exclusively for healthcare professionals. It contains information about a medical device and its clinical application that may require professional background knowledge for proper interpretation.
By clicking “Watch Now”, you confirm that you are a healthcare professional and acknowledge that this content is not intended for the general public.
CATUVAB® – the first MEDICAL DEVICE to remove cancer cells reliably from intraoperative blood during IBS.


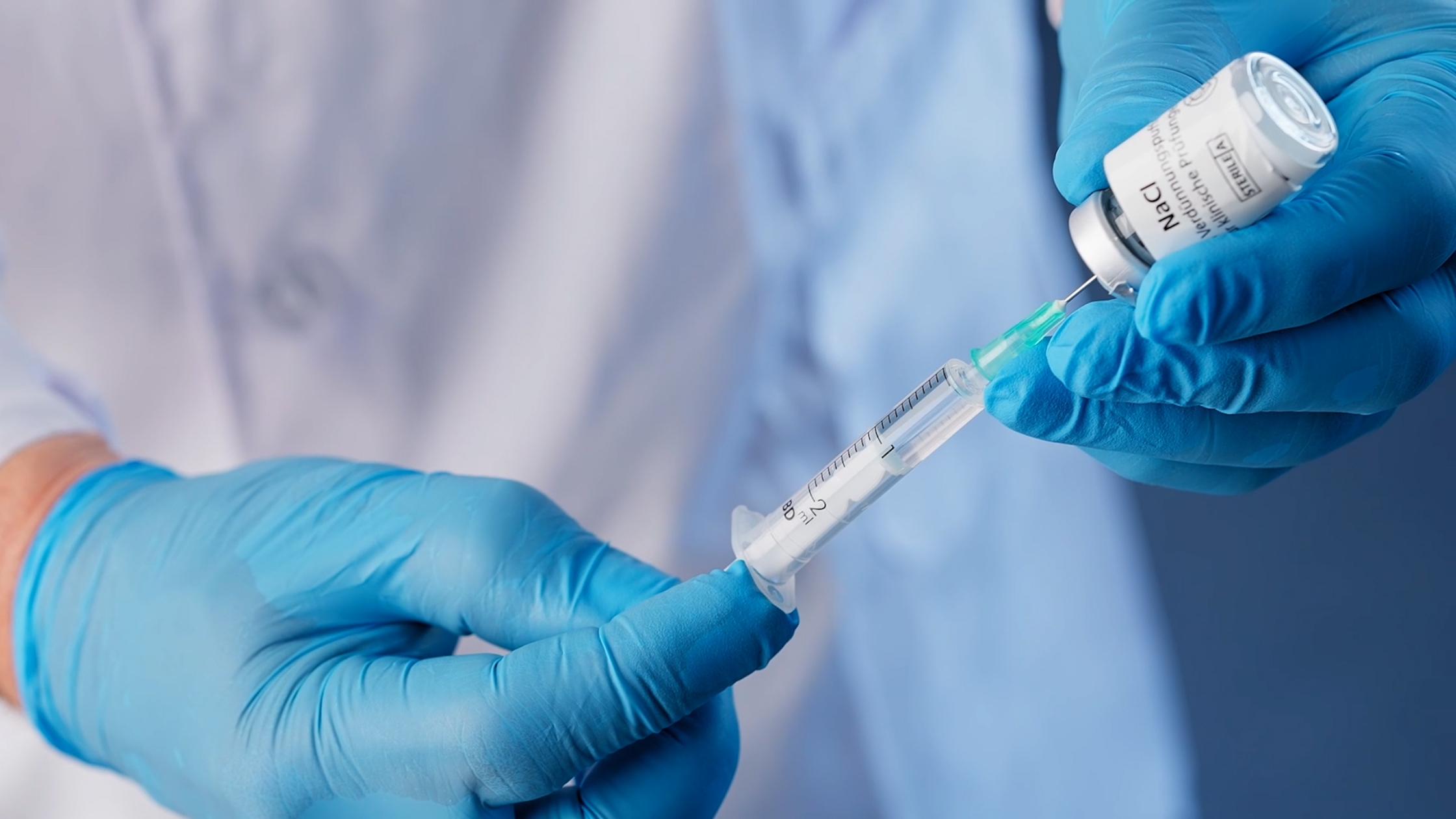
CATUVAB® contains a trifunctional antibody designed to bind and aggregate EpCAM-positive tumor cells and immune cells. These cell complexes are subsequently removed during intraoperative blood salvage. Each kit enables treatment of up to 3 liters of salvaged blood.
The included buffer solution supports optimal antibody activity and is used in conjunction with standard Intraoperative Blood Salvage (IBS/ICS) devices (e.g., Cell Saver systems). It ensures compatibility with routine surgical workflows.
The single-use CATUVAB® kit includes all required sterile syringes and components for safe and efficient application. Designed for seamless integration into standard IBS procedures during EpCAM-positive solid tumor surgeries.
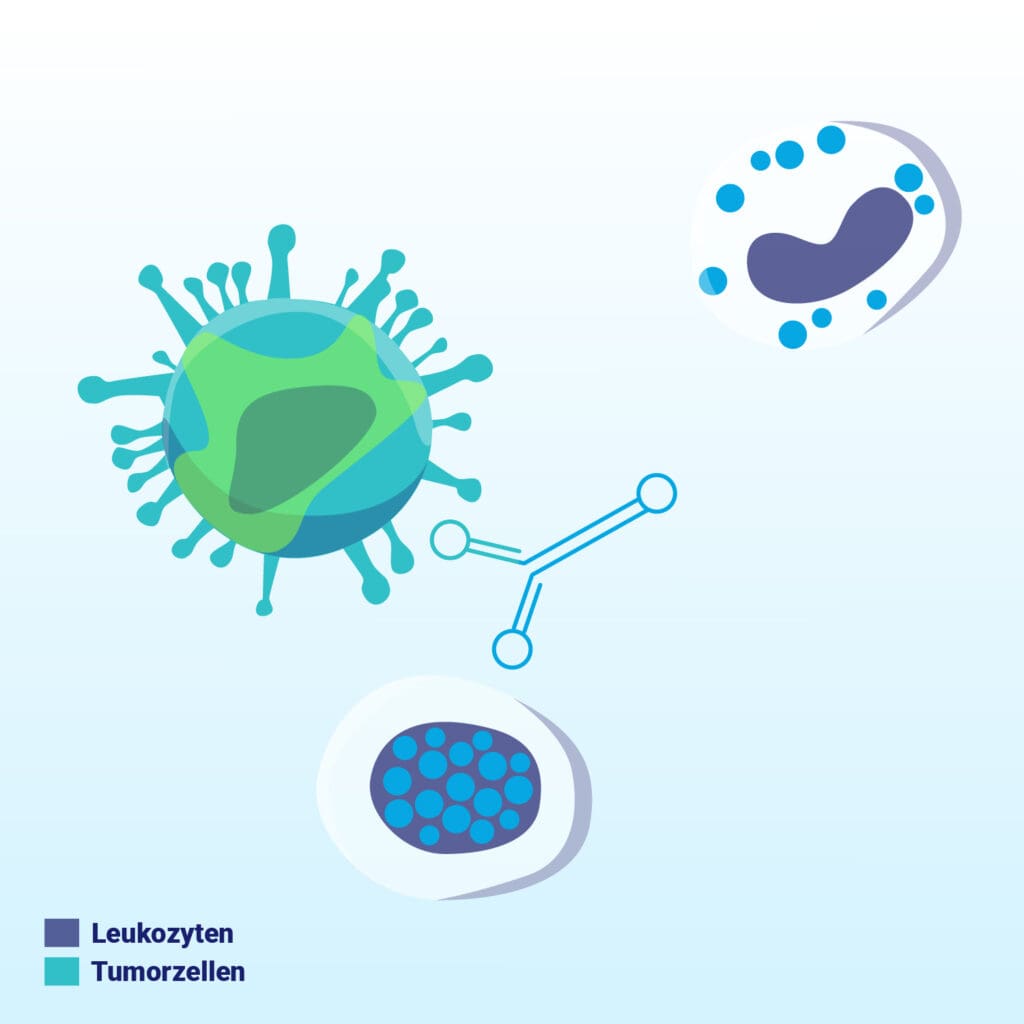
The antibody attaches itself specifically to EpCAM-expressing cells in the patient’s blood – a marker frequently found on tumor cells.
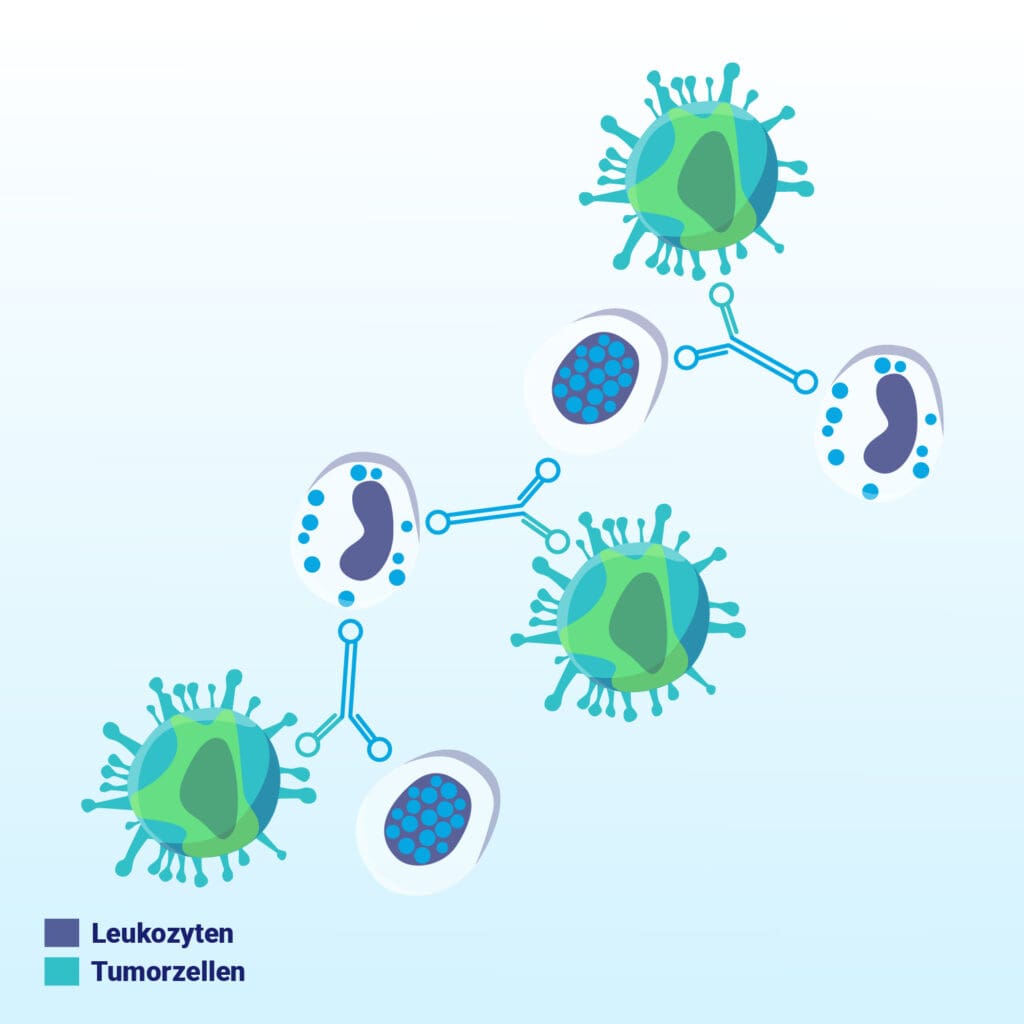
The antibody attaches itself specifically to EpCAM-expressing cells in the patient’s blood – a marker frequently found on tumor cells.
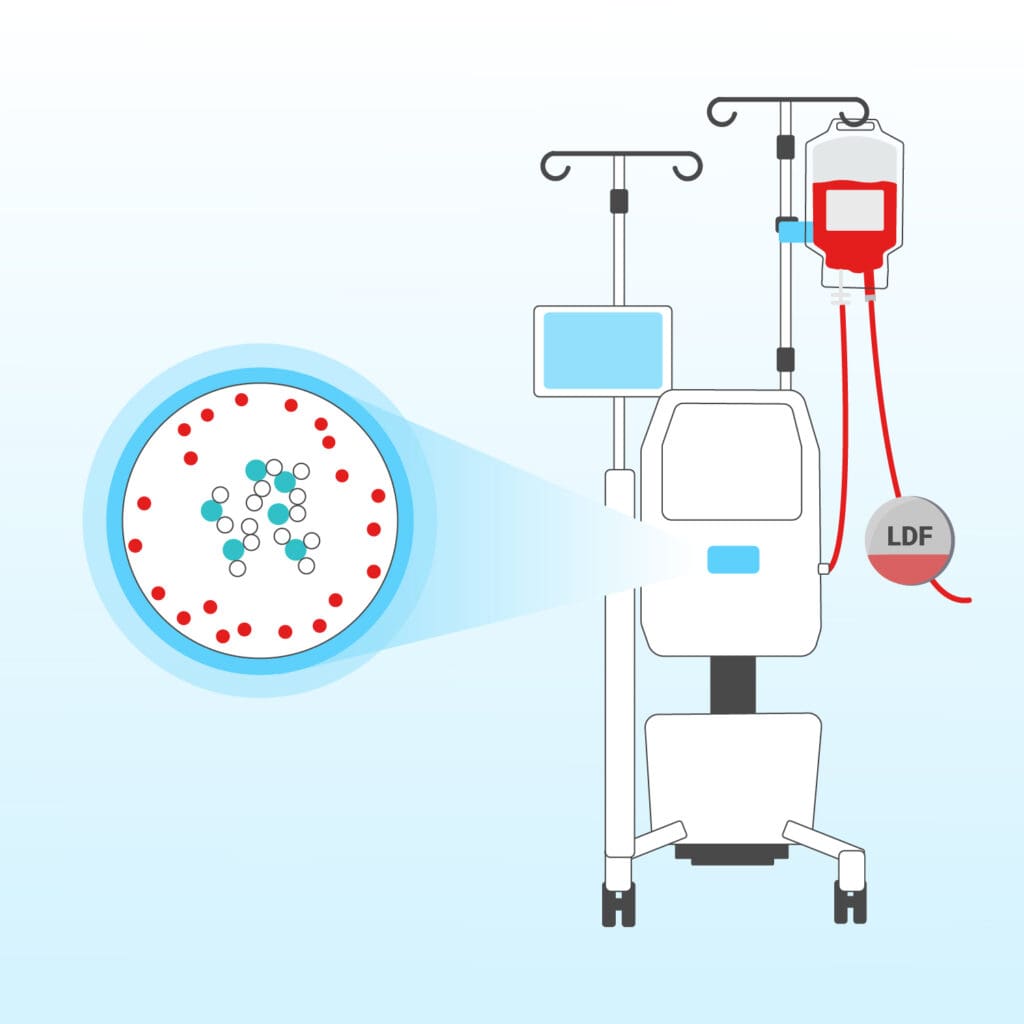
The aggregated tumor cells are retained in the filter of the “Cell Saving” system – leaving only purified, safe blood for retransfusion.
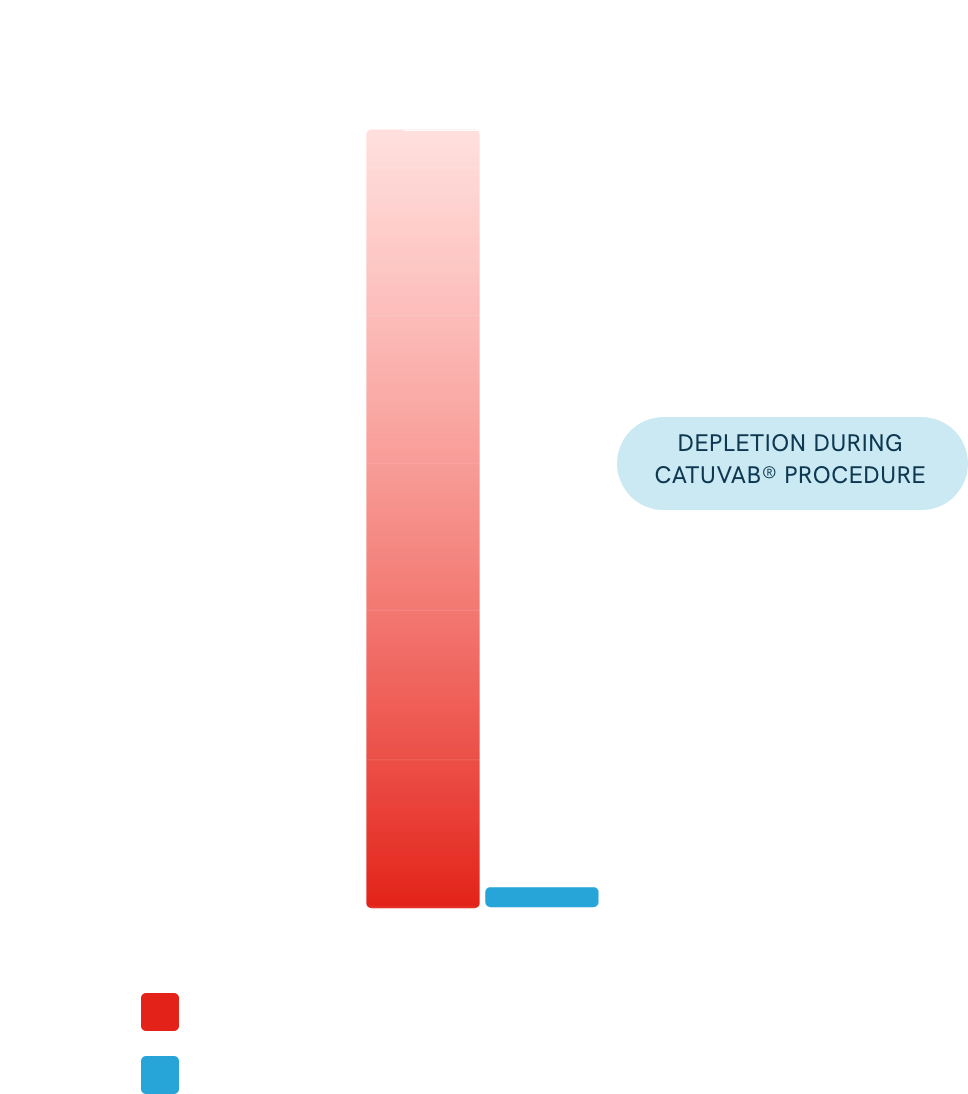
All efficacy endpoints were met with high statistical significance.
In 60 patients with tumor cell–positive blood, 59 had zero residual tumor cells after CATUVAB® treatment.
One patient had only 4 remaining tumor cells.
Up to 4.2 million tumor cells were removed in some cases.
Multi-center study across 6 sites
136 patients evaluated
80 re-transfusions of erythrocyte concentrates
Study endpoints: tumor cell removal, cytokine levels, residual antibody assessments
CE-marked and manufactured under MDR & ISO 13485
Compatible with standard cell saver systems for seamless OR integration
The majority of solid tumors are EpCAM positive of which over 6.4M patients require surgical intervention per year, representing an addressable patient pool for CATUVAB®.
CATUVAB® is compatible with surgeries involving tumors with a high EpCAM+ probability, which are prevalent across many leading cancer types – including breast, colorectal, lung, pancreatic, and prostate cancers.
Autologous blood is immediately available during surgery, eliminating the need for crossmatching or blood bank logistics - especially valuable in urgent oncologic procedures.
By using the patient’s own blood, CATUVAB® significantly lowers the risk of transfusion-transmitted infections and immunologic reactions compared to donor blood.
Autologous transfusion removes the need for blood type compatibility, simplifying perioperative care and reducing procedural complexity.
CATUVAB® enables intraoperative reuse of patient blood, reducing dependency on donor blood supply and lowering overall treatment and storage costs.