CATUVAB®: Using the proprietary ABCR-Technology to aggregate tumor cells and immune cells. CATUVAB is made to overcome the problems with donor blood in cancer surgeries and avoid the consequences of donor blood shortage as e.g. postponing of surgeries.
Our proprietary ABCR Technology uses a trifunctional antibody to selectively bind and aggregate tumor cells and immune cells. These aggregates can then be removed via filtration – enabling safer transfusions in both intraoperative and donor blood applications.
Lindis Blood Care has developed CATUVAB®, a groundbreaking medical device designed to avoid the adverse effects associated with donor blood transfusions during tumor surgeries.
By enabling the safe retransfusion of a patient’s own blood, CATUVAB® enhances patient outcomes and reduces reliance on donor blood.
In major surgical procedures, significant blood loss can occur, often compensated by donor blood transfusions. However, these transfusions carry risks such as immune reactions and the transmission of infections.
Significant blood loss during cancer surgeries often necessitates transfusions.
Donor blood use has serious sideeffects.
Standard blood salvage may reintroduce tumor cells.
CATUVAB® removes tumor cells, enabling safe autologous blood transfusion.
Minimizes risks associated with donor blood transfusion or standard blood salvage.
Intraoperative blood salvage (IBS) enables the recovery and reuse of the patient’s own blood. However, this method is rarely used in oncological surgeries because tumor cells can remain in the blood.
to donor blood transfusion.
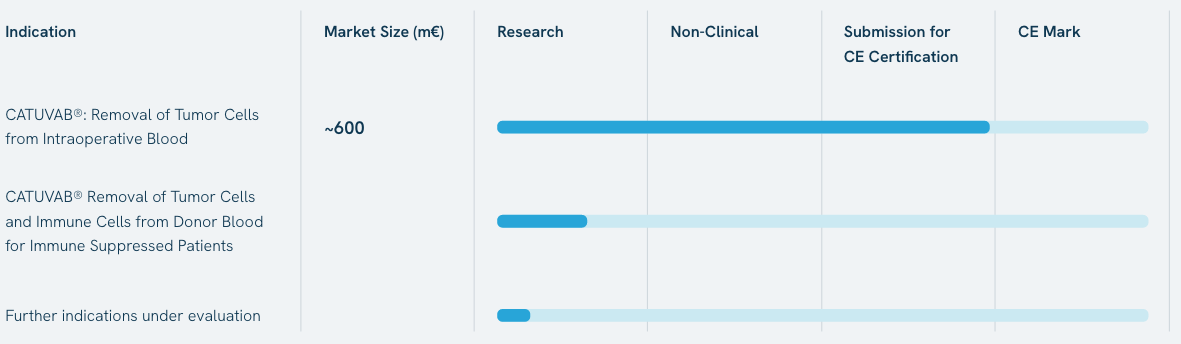
EU-Launch expected for Q4 2025.
Looking for distribution/licensing partners in China, South East Asia and South & North America as well as for Europe excluded the DACH region.
LBC is the MedTech company which developed CATUVAB®, the first device to remove cancer cells and cancer stem cells of 14 major tumor indications from salvaged blood during cancer surgeries
Side effects of allogeneic blood
Access to autologous blood for groups
not allowed to accept allogeneic blood
Plannability of Surgeries

*Shander et al 2007
With our Antibody-Based Cell Removal Technology (ABCR-T), we develop innovative solutions to remove tumor and immune cells from blood — improving patient outcomes and transforming transfusion practices worldwide.
2018, Germany
ABCR-T (Antibody-Based Cell Removal)
EU, US (FDA submited), Asia expansion in preparation
REMOVE study, 136 patients across multiple centers