ABCR‑T is the proprietary and patent protected Antibody-Based Cell Removal Technology of Lindis Blood Care GmbH. It uses a trifunctional antibody to aggregate tumor and/or immune cells, enabling their targeted removal through filtration.
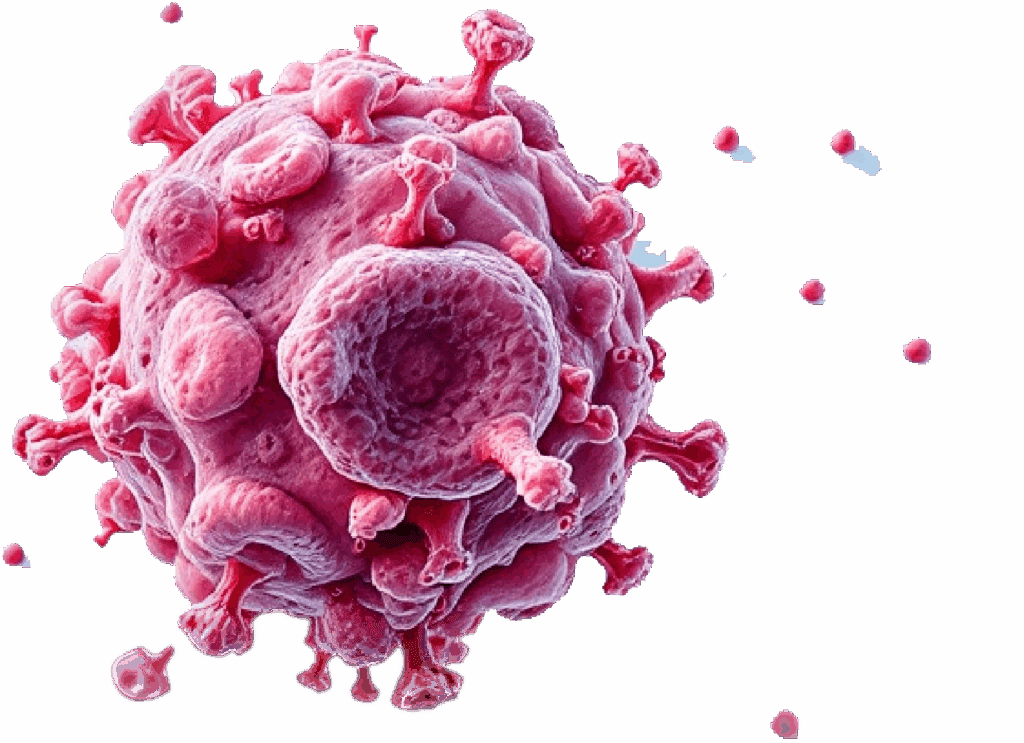
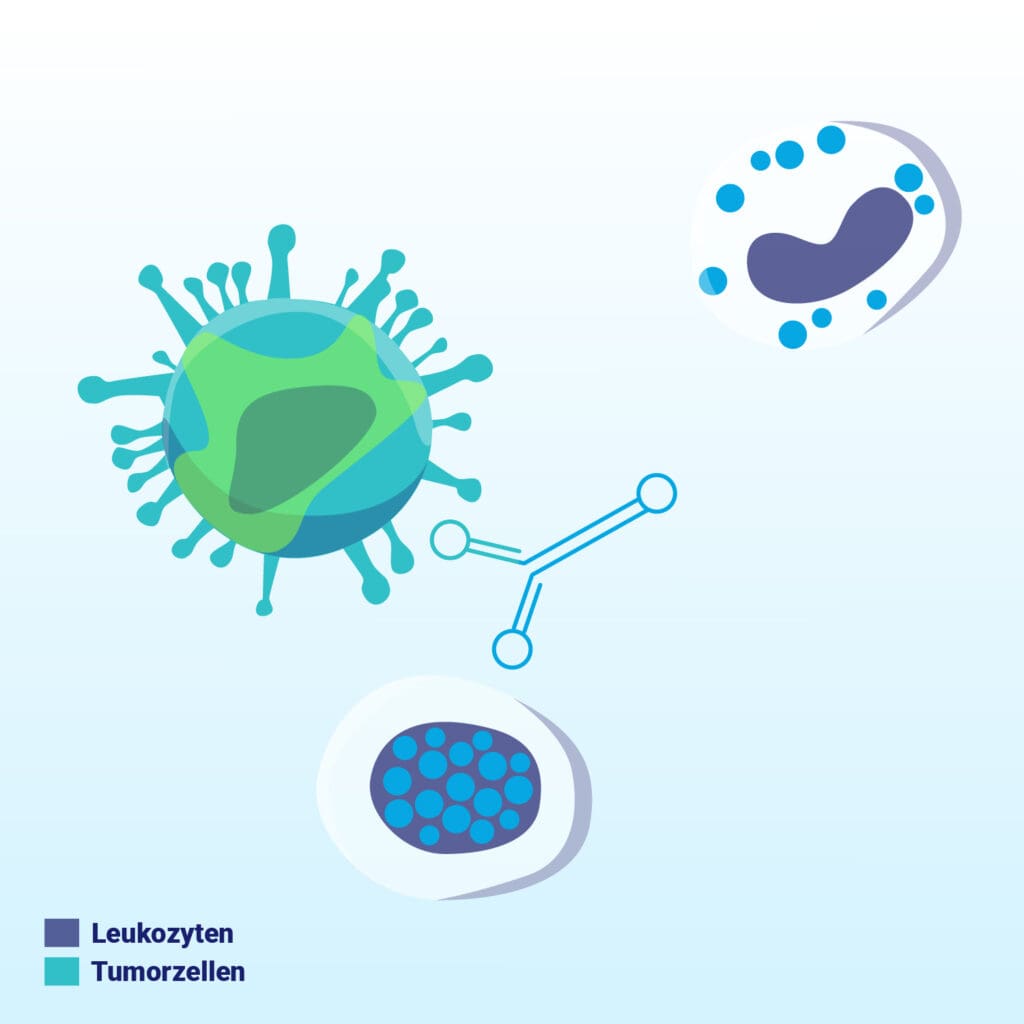
The trifunctional antibody binds to tumor and immune cells in the blood.
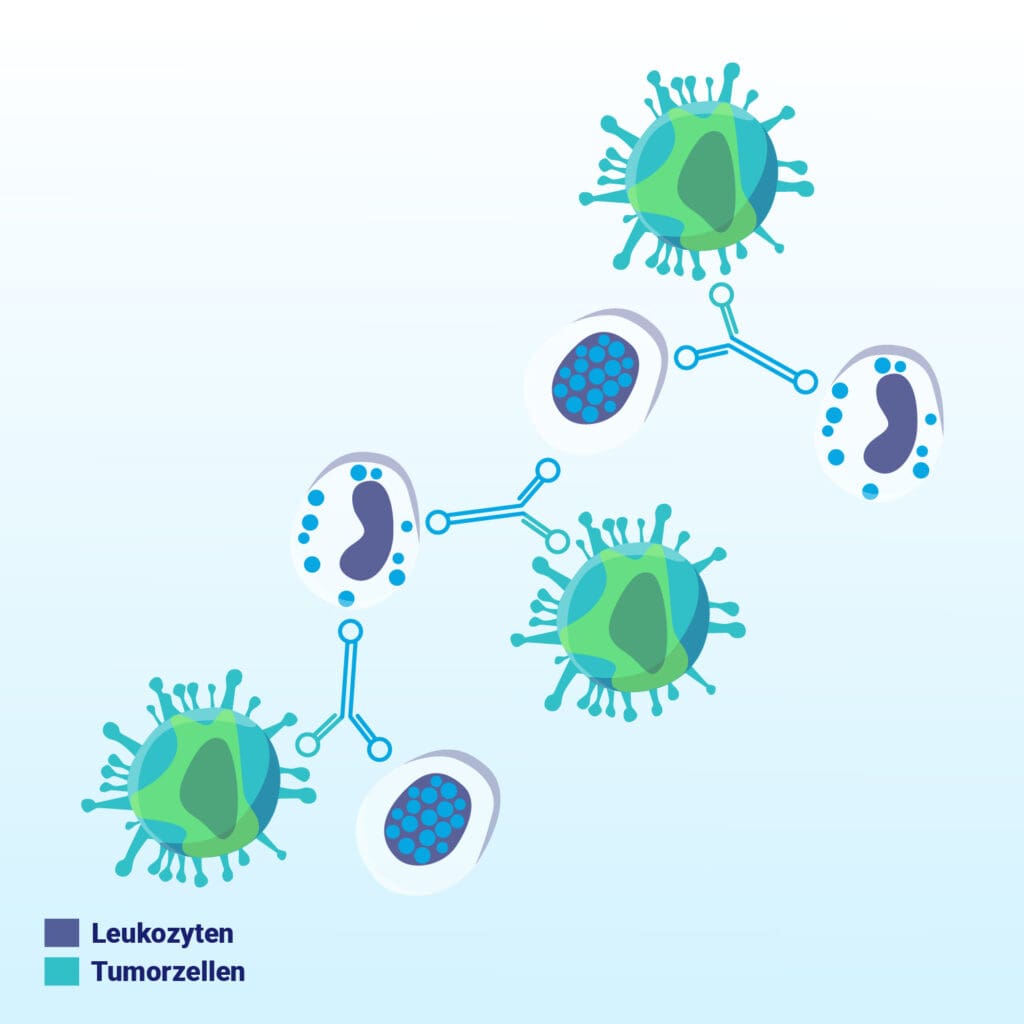
Bound cells cluster together, forming larger cell aggregates.
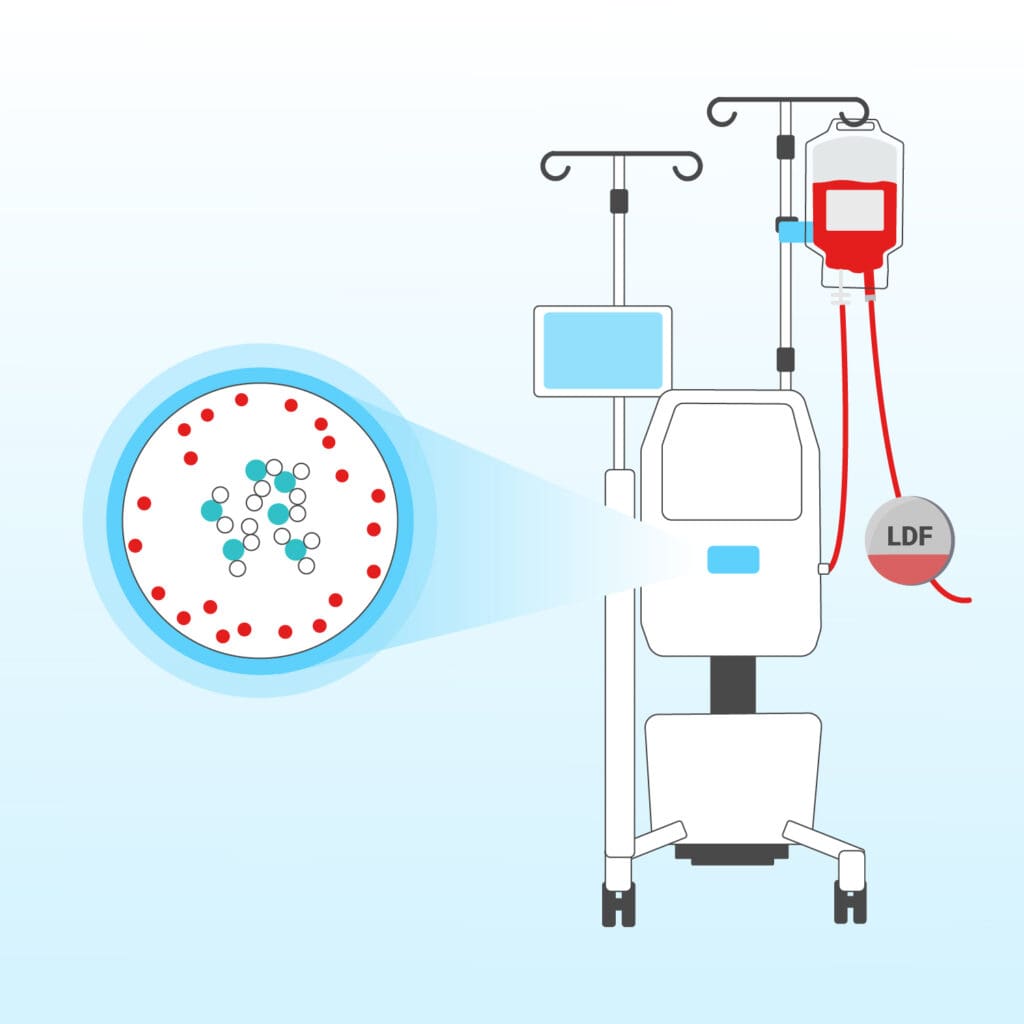
These aggregates are efficiently removed using standard filtration technologies.
INDICATION: Removal of tumor cells from salvaged blood during cancer surgery. Enables safe autologous blood transfusion without irradiation – clinically validated in the REMOVE study.
STATUS: CE Certification process
INDICATION: Removal of tumor and immune cells from donor blood for immunosuppressed patients.
Improves transfusion safety in vulnerable patients and opens new options for prophylactic applications.
STATUS: research / non clinical
INDICATION: Expansion of ABCR Technology to new therapeutic areas. Research is ongoing to explore further applications in oncology and beyond.
STATUS: Research